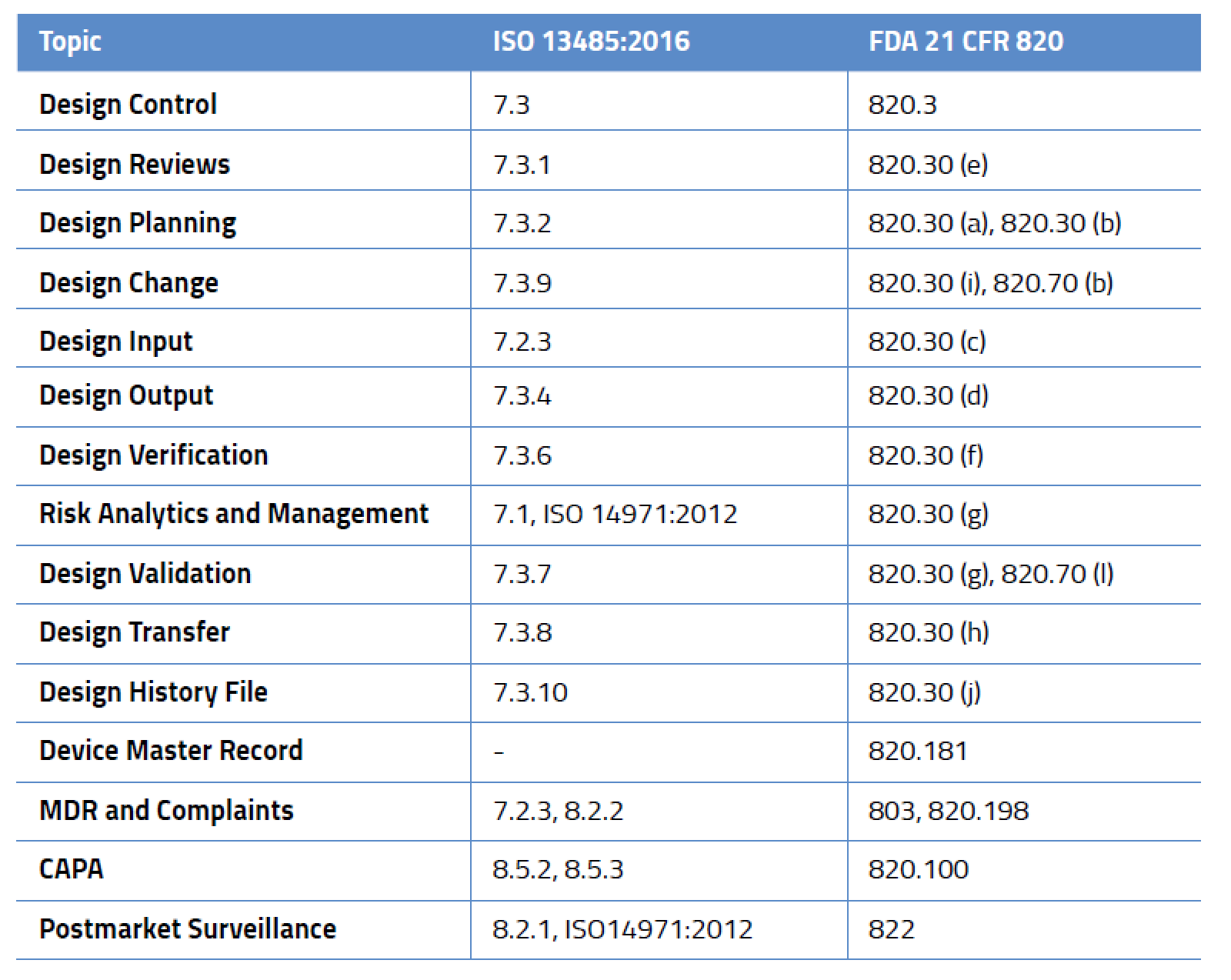
What Are Medical Device Design Controls . safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). this guidance document describes different study design principles relevant to the development of medical device clinical. the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. The design control process follows a set of practices and procedures that help medical product. what are fda design controls: design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process.
from www.orielstat.com
design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. The design control process follows a set of practices and procedures that help medical product. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. this guidance document describes different study design principles relevant to the development of medical device clinical. what are fda design controls:
Basics of Medical Device Design Controls What, Why, and How Oriel STAT A MATRIX Blog
What Are Medical Device Design Controls The design control process follows a set of practices and procedures that help medical product. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. The design control process follows a set of practices and procedures that help medical product. what are fda design controls: design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. this guidance document describes different study design principles relevant to the development of medical device clinical.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies What Are Medical Device Design Controls design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. the us fda identifies design controls that medical device manufacturers must incorporate under their. What Are Medical Device Design Controls.
From www.regdesk.co
Design Controls For Medical Devices An Overview RegDesk What Are Medical Device Design Controls the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. what are fda design controls: The design control process follows a set of practices and procedures that help medical product. design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process.. What Are Medical Device Design Controls.
From www.slideteam.net
Medical Device Design Control Cycle Ppt Powerpoint Presentation Layout Cpb Presentation What Are Medical Device Design Controls this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp).. What Are Medical Device Design Controls.
From www.youtube.com
Design Control for Medical Devices Online introductory course YouTube What Are Medical Device Design Controls the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. The design control process follows a set of practices and procedures that help medical product. this documentation process is widely. What Are Medical Device Design Controls.
From www.plm.automation.siemens.com
An Intelligent Approach to Design Control for Medical Devices Siemens Software What Are Medical Device Design Controls the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. this guidance document describes different study design principles relevant to the development of medical device clinical. what are fda design controls: this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet. What Are Medical Device Design Controls.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies What Are Medical Device Design Controls the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. this guidance document describes different study design principles relevant to the development of medical device clinical. what are fda design controls: safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp).. What Are Medical Device Design Controls.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel STAT A MATRIX Blog What Are Medical Device Design Controls this guidance document describes different study design principles relevant to the development of medical device clinical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). what are fda design controls: this documentation process is widely known as “design controls” and its purpose is to ensure that. What Are Medical Device Design Controls.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide What Are Medical Device Design Controls the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. The design control process follows a set of practices and procedures that help medical product. safe medical device act. What Are Medical Device Design Controls.
From kalypso.com
Design Controls for Med Device Kalypso What Are Medical Device Design Controls this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. this guidance document describes different study design principles relevant to the development of medical device clinical. The design control process follows a set of practices and procedures that help medical product. design controls for medical. What Are Medical Device Design Controls.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies What Are Medical Device Design Controls safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). The design control process follows a set of practices and procedures that help medical product. the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. what are fda design controls: design. What Are Medical Device Design Controls.
From www.cognidox.com
Medical Device Development Guide What Are Medical Device Design Controls The design control process follows a set of practices and procedures that help medical product. the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. safe medical device act of. What Are Medical Device Design Controls.
From www.scribd.com
Presentation Medical Device Design Controls PDF Medical Device Verification And Validation What Are Medical Device Design Controls safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. this guidance document describes different study design principles relevant to the development of medical device clinical.. What Are Medical Device Design Controls.
From operonstrategist.com
FDA Design Control The Ultimate Guide For Medical Device Companies Operon Strategist What Are Medical Device Design Controls safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). The design control process follows a set of practices and procedures that help medical product. this guidance document describes different study design principles relevant to the development of medical device clinical. the us fda identifies design controls that. What Are Medical Device Design Controls.
From www.qualio.com
Ultimate guide to medical device design controls What Are Medical Device Design Controls The design control process follows a set of practices and procedures that help medical product. the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. what are fda design controls:. What Are Medical Device Design Controls.
From operonstrategist.com
US FDA 21 CFR 820.30 (Design Controls For Medical Devices) Operon Strategist What Are Medical Device Design Controls safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). this guidance document describes different study design principles relevant to the development of medical device clinical. design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. . What Are Medical Device Design Controls.
From www.elexes.com
Medical Device Design & Development Guide! What Are Medical Device Design Controls the us fda identifies design controls that medical device manufacturers must incorporate under their qms when. design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user. What Are Medical Device Design Controls.
From docslib.org
The Ultimate Guide to Design Controls for Medical Device Companies DocsLib What Are Medical Device Design Controls The design control process follows a set of practices and procedures that help medical product. this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process.. What Are Medical Device Design Controls.
From operonstrategist.com
US FDA 21 CFR 820.30 (Design Controls For Medical Devices) Operon Strategist What Are Medical Device Design Controls design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and development process. this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended. this guidance document describes different study design principles relevant to the development of medical. What Are Medical Device Design Controls.